Back on the Table Element 118 Is Served Up Again Element 118 Name
The periodic table of the elements is one of mankind's greatest discoveries in nature since information technology encompasses all the edifice blocks that bind our universe together at its eye: from the tiniest virus to the most distant galaxy. Last time, nosotros discussed the difficult road towards element 118. In this office, nosotros have a await at the start synthesis of element 118, its properties, and how new elements are named.
5. Beginning Bodily Synthesis of Chemical element 118
In 2002, a enquiry group consisting of scientists from the Joint Institute for Nuclear Research in Dubna, Russia, together with the Lawrence Livermore National Laboratory in Berkeley, CA, USA, commenced with the first synthetic attempt to produce element 118 by battery of californium-249 with calcium-48 ions [21–23]. Calcium-48, with a natural affluence of simply 0.nineteen %, is very rare, and correspondingly plush (USD 200,000/g). For a light element (Z = 20), it is extraordinarily neutron-rich with a neutron count of 28, and for that reason, especially well-suited to the synthesis of stable, heavy nuclei.
For 100 days, the team bombarded a target consisting of 10 mg of 249Cf (0.23 mg/cm2) with a calcium-48 beam of 2·x12 roughly 17-fold positively-charged ions per 2nd; in the class of iii months of irradiation, a total of two·1019 calcium ions. During this entire menstruation, they obtained evidence for merely a single disuse sequence attributable to element 118!
48 xxCa +249 98Cf → 297[118]
Work was resumed in 2006, revealing, as required, two additional relevant disuse sequences [24] (meet Fig. 3). This time, numerous prerequisites had to be fulfilled for recognition of the discovery, published in detail by the International Union of Pure and Applied Chemistry (IUPAC) and the International Union of Pure and Applied Physics (IUPAP) [25, 26]. Sufficient disuse series consistent with chemical element 118 were indeed established, but none of the isotopes involved were previously known. In that location was, thus, a failure to create a link to known isotopes, so recognition had to exist denied [27]: "The three analyzed disuse series for an isotope of element Z = 118 are in adept mutual understanding, simply in the absence of anchoring to known nuclei, the requisite criteria for recognition are not fulfilled."
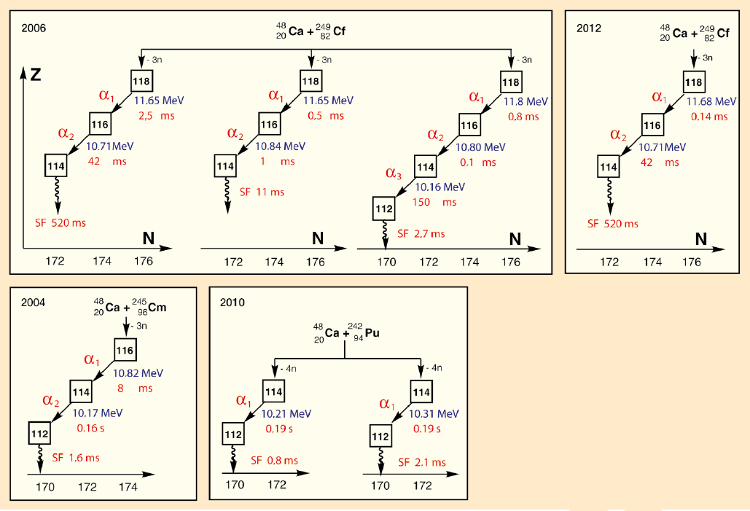 |
| Effigy 3. Experiments relevant to the discovery of element 118. |
It was possible, however, to confirm the decay series for element 118 via independent studies involving lateral entries. Thus, elements 116 and 114 were prepared via independent paths by bombardment of curium and plutonium, respectively, with calcium-48 ions, and their decay serial were determined (see Fig. 3). These decay series were found to be consequent with the respective parts of the decay series for element 118. Every bit a consequence, IUPAC/IUPAP indeed released a positive judgment:
"The 2006 Dubna–Livermore collaboration of Oganessian et al. produced three concordant decay chains commencing with 294118. This result was confirmed in 2012. Three other independent heavy element fusion studies served to identify and confirm the existence and decay properties of 294118 descendants 290Lv and 286Fl serving to link atomic numbers through cross bombardments. The Dubna–Livermore 2006 collaboration has satisfied the criteria for discovery and its claim is at present acknowledged as validated."
Thus, information technology was officially recognized that the Russian–American enquiry group under the direction of Yuri Oganessian had indeed discovered chemical element 118. The President of the IUPAC Inorganic Segmentation then requested an appropriate proffer for a name and symbol for the new chemical element. Consistent with boosted regulations, the plenary session of IUPAC voted on the acknowledgment of the discovery and the naming of element 118.
vi. The Naming of the Elements 113–118
The discoverers of an element take the right to suggest a proper name for information technology, but they do non have a completely gratuitous option. IUPAC and IUPAP have developed certain applicable rules [28, 29], and the name of the new element is to exist derived from:
- a) a mythological concept or character, including astronomical objects
- b) a mineral or similar material
- c) a place or geographical region
- d) a characteristic of the chemical element
- e) the name of a scientist
In the interest of standardization, suggested element names for certain elements should always have one of the following endings:
- Groups 1–16, including elements of the f-block: "-ium"
- Grouping 17 (halogens): "-ine"
- Group 18 (noble gases): "-on"
Decisions regarding chemical element names are difficult for all the parties involved: non simply the discoverer, just also IUPAC/IUPAP. Discoverers, with their ain egos, necessarily live in a specific complex political environment, and IUPAC/IUPAP and their international experts likewise do not exist in a vacuum. In the Cold War era, this led to disputes that have sometimes been quite grotesque. Thus, over a menses of three decades, element 104 was identified in schoolbooks in the Usa as rutherfordium, only in Russian as kurchatovium. Fortunately (hopefully?), this period is over. Today, the synthesis of the heavy transactinides shows how advantageous it is when research groups from unlike countries employ their expertise to common projects.
6.1. Element 113: Nihon (Nh)
Element 113 was first prepared—afterward years of effort—by a Japanese group at RIKEN led by Kosuke Morita. The suggested proper noun and symbol were designed to honor their homeland: "Nihon" (land of the ascent dominicus), with the accompanying symbol Nh.
Morita and his squad began bombarding a bismuth target with zinc ions in 2003, and by April 2005, they had detected two consistent decay series for chemical element 113. This was non considered sufficient for recognition, however. Only in August 2012, after seven additional years of irradiation, was the long-yearned-for third decay serial observed. With their countless patience and tenacity, the research group showed the limit of today'southward technical possibilities: one decay series in two years!
Preparation of the three other elements recognized in 2017—numbers 115, 117, and 118—was the result of a close collaboration between Russian (Dubna) and US (Berkeley) scientists. This had the further advantage that the parties involved were able to agree on an amicable compromise with regard to the matter of naming in the preliminary stages.
6.2. Element 115 – Moscovium (Mc)
The Russian capital was honored with the name moscovium (Mc), just as had already been the example for the research site Dubna, only 100 km abroad from Moscow, with chemical element 105 (dubnium).
6.three. Element 117 – Tennessine (Ts)
The ending "ine" reveals that this chemical element is associated with the 17th group in the periodic table: the halogens (fluorine, chlorine, bromine, etc.). The suggested proper noun was surprising for many, since element 117's discovery was attributed to the Dubna/Berkeley team of Yuri Oganessian. Merely a closer look at the synthesis explains the background:
48 20Ca +249 97Bk → 293[117] + 4n
The battery of berkelium-249 with calcium-48 ions had been planned past Oganessian'south team for a long fourth dimension. The problem was that there was only one place on Earth where a few milligrams of the required berkelium might exist prepared: in the high-flux reactor at the Oak Ridge National Laboratory (ORNL) in the state of Tennessee, USA [30, 31]. There ane could actually purchase 249 97Bk, admitting at a price of USD 185 per microgram (!), non including packaging. The planned experiment required 20 mg. It was necessary to take into business relationship not only the high cost, but as well the short half-life of 249 97Bk: only 330 days. A comprehensive American-Russian masterplan was developed for the preparation of chemical element 117, roofing all aspects of the timing of the experiment itself, too as further processing and send over thousands of kilometers.
In the leap of 2008, 40 g of curium-244 was introduced into the ORNL high-flux reactor, and subjected to extremely loftier neutron irradiation for 23 days. After the fuel was wearied, it was replaced, and the curium sample was irradiated for another 23 days. Following eleven such cycles over a total of 250 days, 22 mg of 249 97Bk had been prepared, and over the side by side six months, it was isolated from the curium sample and then purified (see Fig. four).
 |
| Figure 4. The starting material for the tennessine synthesis: berkelium-249. |
A solution of the resulting berkelium chloride was placed in 5 pb receptacles and transported to Moscow on a commercial flying. The collaborative efforts between the researchers had proceeded without problems, however, the aforementioned could not exist said with respect to subsequent sample transport. Twice, the berkelium containers were refused at the Russian edge because of missing or incomplete documents, so sent back to New York. Only on the tertiary try did they reach the Russian Research Plant for Diminutive Reactors in Dimitrovgrad, where the target discs were prepared. Finally, the actual experiment could brainstorm in Dubna on July 27, 2009. A first disuse serial of element 117 was found on Baronial xx, 2009, and over the class of the next half dozen months, v additional decay serial were recorded.
6.four. Element 118 – Oganesson (Og)
The ending "-on" implies that this chemical element is recognized as one of the "noble gases" (group eighteen). The name assigned to information technology is a tribute to Yuri Oganessian (encounter Fig. 5), who became just the second living scientist for whom an element was named after Glenn T. Seaborg with seaborgium. When asked what his feelings were well-nigh that, Oganessian replied [23]:
"For me, it is an honor. Discovery of element 118 was accomplished past scientists from the Joint Constitute for Nuclear Research in Russia and the Lawrence Livermore National Laboratory in the United States, and it was my colleagues who suggested the proper name oganesson. My children and grandchildren accept been living in the United for decades already, simply my girl wrote to me when she heard about it that she was unable to sleep that night considering she was crying so much. My grandchildren, in contrast, similar all young people, barely reacted."
 |
| Figure 5. Yuri Oganessian on an Armenian stamp. |
For chemists, the question arises whether oganesson is not just formally a noble gas, but whether information technology besides behaves physically and chemically like ane. Many chemists remain skeptical nigh quantumchemical calculations, especially with respect to the transactinides, since the high velocities of their inner electrons (70 % of the speed of calorie-free in the case of copernicium, Z = 112) are more than difficult to accept into account (relativistic furnishings). But if Dmitri Mendeleev was able to brand certain precise and correct predictions even in the 19th century using but pencil and paper, nosotros should maybe place more trust in our theoreticians. In whatever case, based on their calculations, the opening question "Is element 118 a noble gas?" tin quickly be answered by "Oganesson is guaranteed not to exist a noble gas, merely perhaps rather a "noble liquid", with a boiling point of 50–110 °C [32].
Moreover, oganesson should react with fluorine to give the stable compounds OgF2 and OgFfour [33, 34], where OgFfour would not be planar similar xenon tetrafluoride, just tetrahedral. This cannot still be verified experimentally, since the four synthetic oganesson atoms were gone later on a single millisecond. Let u.s., therefore, look toward more stable oganesson isotopes, and prepare to be surprised by their chemistry.
7. How Do Things Go on from Here?
With respect to the synthesis of element 119, nuclear researchers are still at the starting gates. Hideto En'yo from the Japanese research institute RIKEN has announced a bombardment of curium with vanadium ions
96Cm + 23V → [119]
and the Oganessian group wants to fire titanium ions at berkelium
97Bk + 22Ti → [119]
It is nearly sure that these experiments will bump upwardly confronting the limits of current applied science. The synthesis of yet heavier elements certainly presupposes additional major technical developments. We will take to come across over what fourth dimension catamenia the necessary progress will exist achieved. We, therefore, wish our nuclear scientists both good ideas and expert luck in their further search for new elements. However, not too much luck, since if they were all of a sudden to stumble across stable isotopes, no one would even observe it.
Nosotros mustn't forget that the heavy nuclei are so far recognizable only on the basis of their radioactive tracks. So if stable nuclei were to emerge, nosotros wish nuclear scientists simultaneously the technical possibility of preparing weighable amounts of the corresponding elements. Then one might be able to study chemical reactions of their electron shells. And that would exist exciting since, for instance, theoretical computations propose that chemical element 123 should possess three partially-filled orbitals in three different shells (8sii 8p 7d 6f). Nosotros should be specially anxious to study element 125, considering it will involve occupied g-orbitals for the commencement fourth dimension ([Og] 8s2 8p 6fthree 5g). Merely the forms of the various 5g-orbitals cause a desire to examine their chemistry (see Fig. 6). Nosotros will expect expectantly!
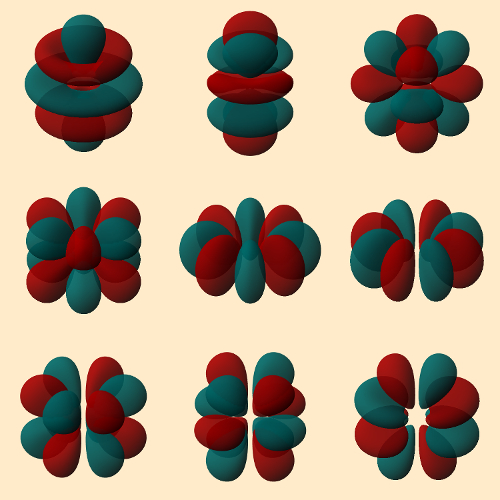 |
| Figure 6. The 5g orbitals; from left to right and from top to lesser [35]: |
References
[21] Y. T. Oganessian et al., Results from the First 249Cf+48Ca Experiment, Lawrence Livermore National Laboratory Report 2003.
[22] Y. T. Oganessian, Superheavy elements, Pure. Appl. Chem. 2004, 76, 1715. https://doi.org/10.1351/pac200476091715
[23] R. Greyness, Mr Element 118: The only living person on the periodic table, New Scientist 2017, April 15, 40.
[24] Y. T. Oganessian et al., Synthesis of the isotopes of elements 118 and 116 in the 249Cf and 245Cm+48Ca fusion reactions, Phys. Rev. C 2006, 74, 044602. https://doi.org/10.1103/PhysRevC.74.044602
[25] A. H. Wapstra, Criteria that must be satisfied for the discovery of a new element to be recognized, Pure Appl. Chem. 1991, 63, 879. https://doi.org/x.1351/pac199163060879
[26] R. C. Barber et al., Discovery of the transfermium elements, Pure Appl. Chem. 1993, 65, 1757. https://doi.org/x.1351/pac199365081757
[27] P. J. Karol et al., Discovery of the element with diminutive number Z = 118 completing the 7th row of the periodic tabular array, Pure Appl. Chem. 2016, 88, 155. https://doi.org/ten.1515/pac-2015-0501
[28] W. H. Koppenol, Naming of new elements (IUPAC Recommendations 2002), Pure Appl. Chem. 2002, 74, 787. https://doi.org/10.1351/pac200274050787
[29] W. H. Koppenol et al., How to proper noun new chemical elements (IUPAC Recommendations 2016), Pure Appl. Chem. 2016, 88, 401. https://doi.org/10.1515/pac-2015-0802
[30] J. S. Bardi, An Cantlet At The Finish Of The Textile World, Inside Science 2010, April 8.
[31] K. Chapman, What it takes to make a new element, ChemistryWorld 2017, January 22.
[32] C. S. Nash, Atomic and Molecular Properties of Elements 112, 114, and 118, J. Phys. Chem. A 2005, 109, 3493. https://doi.org/x.1021/jp050736o
[33] Y.-K. Han, Y. S. Lee, Structures of RgF n (Rg = Xe, Rn, and Element 118. n = 2, iv.) Calculated by Two-component Spin−Orbit Methods. A Spin−Orbit Induced Isomer of (118)Ffour, J. Phys. Chem. A 1999, 103, 1104. https://doi.org/10.1021/jp983665k
[34] Grand. Due south. Pitzer, Fluorides of radon and element 118, J. Chem. Soc. Chem. Comm. 1975, 760. https://doi.org/x.1039/C3975000760B
[35] One thousand. Winter, The Orbitron, wintertime.group.shef.ac.britain.
The article has been published in German as:
- Ist das Chemical element 118 ein Edelgas?,
Klaus Roth,
Chem. unserer Zeit 2017, 51, 418–426.
https://doi.org/x.1002/ciuz.201700838
and was translated by Due west. E. Russey.
New Kids on the Table: Is Element 118 a Noble gas? – Part ane
The synthesis of heavy elements
New Kids on the Table: Is Element 118 a Noble Gas? – Part 2
The difficult road towards element 118
New Kids on the Tabular array: Is Element 118 a Noble Gas? – Part 3
The first synthesis of element 118, its properties, and naming new elements
See similar articles past Klaus Roth published in ChemViews Mag
Source: https://www.chemistryviews.org/details/ezine/11046703/New_Kids_on_the_Table_Is_Element_118_a_Noble_Gas__Part_3/
0 Response to "Back on the Table Element 118 Is Served Up Again Element 118 Name"
Post a Comment